Liver Exchange: A Pathway to Increase Access to Transplantation
Saad Salman, Sarah Gurev, Muhammad Arsalan, Faisal Dar, & Alex Chan
Abstract
We illustrate how paired liver exchange can be used to circumvent living donor-recipient ABO incompatibilities. Specifically, we show how a well-designed liver exchange algorithm can substantially and ethically expand the legal donor pool for incompatible living liver donor‐recipient pairs in South Asia and beyond.
Introduction
For people suffering from liver cancer or cirrhosis, a transplant is often the only definitive treatment option. Approximately 30-50% of these patients are unable to find a donor, and in Pakistan nearly 10,000 people die annually waiting for a liver.1 In this paper, we will outline how the use of a novel algorithm for Liver Paired Donation (LPD) can increase live donor matches in a manner that is safe, ethical, and accessible. We also identify current efforts and barriers to widespread implementation of LPD in Pakistan.
From Kidney Exchange to Liver Exchange?
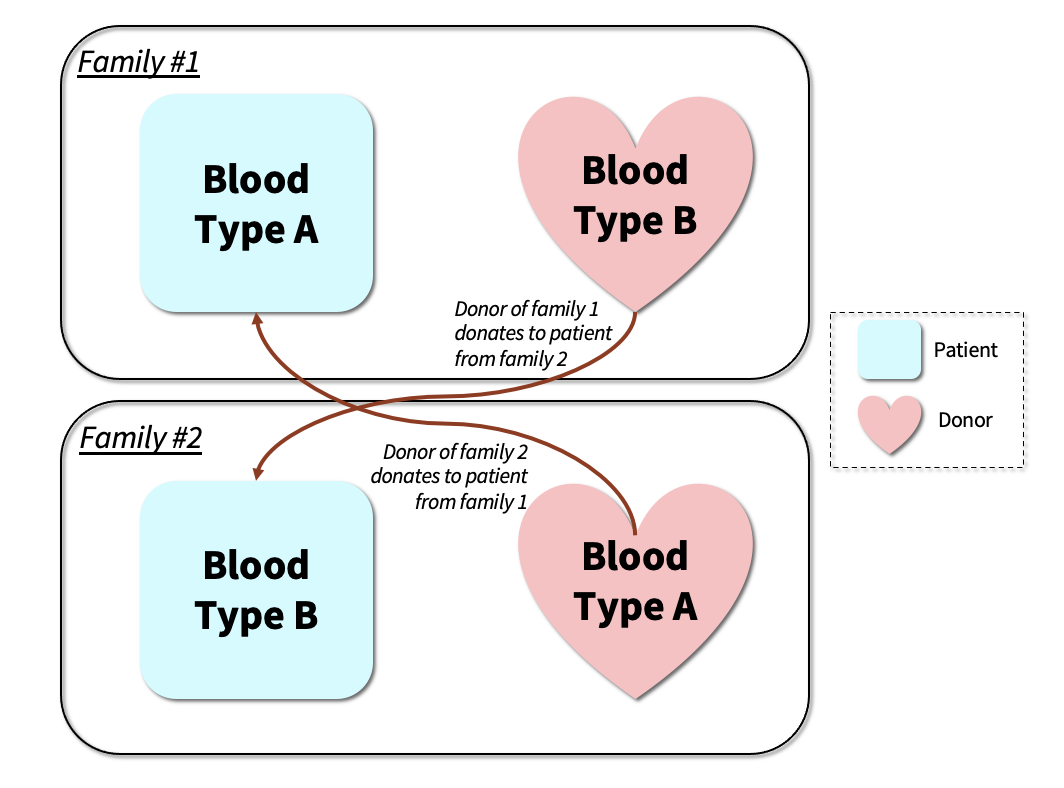
Kidney transplant centers around the world employ the use of a Nobel Prize winning kidney paired donation (KPD) algorithm which allows for mutually beneficial swaps between patient-donor pairs.2,3 KPD circumvents compatibility issues seen with direct donations. For example, a direct donation would be impossible between a recipient with blood type A+ with a B+ donor. However, if we were to find a second pair with a B+ recipient and an A+ donor, the B+ donor from the first pair can donate to the B+ recipient in the second pair in exchange for the A+ donor in the second pair donating to the A+ recipient in the first pair (see Figure 1). KPD has increased the pool of compatible living donors, substantially expanding access to life saving organs and becoming the fastest growing source of transplantable kidneys since the early 2000s.4
Figure 1. Kidney exchange is an innovative way to increase transplants. The idea is to find exchanges between pairs of patients and donors. In the example, suppose a willing donor in family #1 is willing to donate a kidney to the patient in family #1 but is incompatible, and the donor and patient in family #2 are in an identical position. It may be possible to transplant family #1's donor’s kidney to family #2's patient and family #2's donor's kidney to family #1's patient. Such a two-way swap enables both patients to receive a transplant, with each patient’s related donor only donating one of their kidneys.
Similar solutions for liver transplantation, however, have rarely been attempted.5 Livers for transplantation are sourced either from a cadaver (Deceased Donor Liver Transplant or DDLT) or a living donor (Living Donor Liver Transplant or LDLT). In the United States and Europe, the vast majority of transplants are DDLT. Globally, however, over 90% of all liver transplants are LDLT, in part due to the strong social stigma surrounding deceased organ donation in many Asian countries. This reliance on living donors exacerbates organ scarcity, which drives patients to unregulated black markets, creating ethical challenges in addition to jeopardizing the safety of donors and recipients alike.
Paired donor LDLT remains scarce due to two major obstacles to applying KPD-style algorithms to LPD.6 First, while kidney donors donate an entire kidney, the liver is composed of two lobes of which either the smaller left lobe or the larger right lobe is used for LDLT. Compared to the left lobe, resecting the larger right lobe carries a five-fold risk of postoperative complications for the donor.7 However, left lobe grafts may not always be sufficient for recipients with severe liver disease due to their smaller volume. This discrepancy means that in a paired exchange consisting of a left lobe donor and a right lobe donor, the latter will incur a higher health risk, creating inequity across donors. For example, it would be unfair for a recipient whose blood relative has donated a high risk, large volume right lobe to the organ pool to in turn receive a smaller volume left lobe. This disparity in safety and efficacy complicates LPD and requires that LPD algorithms factor in the risk of lobe mismatching.
The second obstacle is the difference in urgency for liver versus kidney transplantation. Kidney transplant candidates can undergo dialysis, a procedure that mimics the role of the kidneys and thus delays disease progression until patients can receive an available donor kidney. There is no similar life-extending intervention available to liver transplant candidates, meaning that while the primary objective of KPD is to maximize the number of swaps, LPD must prioritize swaps based on potential patient mortality. Globally, transplant centers prioritize transplant recipients by using the MELD score (Model for End-Stage Liver Disease), which predicts how likely a patient will die without a liver transplant. Given the difference in patient prioritization for transplantation, algorithms suitable for kidney exchange cannot be applied to liver exchange.
Using a Novel Algorithm to Establish Pakistan as a Pioneer of Liver Exchange
In the first week of November 2020, there were 31 patients waiting for a donor liver at the Quaid-e-Azam International Hospital (QIH), a major liver transplant outfit in Pakistan. Only 17 of these patients had a compatible donor while the remaining 14 patients did not. We sought to maximize the number of compatible donors while also prioritizing recipients with the highest MELD scores. By using earlier LPD algorithms, we were only able to produce 2 additional matches, neither of which had a high MELD score.8 However, when we applied the Stanford algorithm for LPD, we were able to produce an additional 5 patient-donor pairs (29.4% increase) while maintaining priority based on MELD scores (see Figure 2).1 In addition to creating more matches, the Stanford algorithm addressed the issue of lobe mismatching by ensuring that all recipients received the same type of lobe that their relative donated to the exchange pool, thus preventing donor inequity. For these reasons, we believe the Stanford algorithm can serve as a basis for liver exchange around the world, including the United States.
Figure 2: Liver exchange in Pakistan using the Stanford Algorithm at the end of 2020. In the first week of November 2020, there were 31 patients waiting for a donor liver at the Quaid-e-Azam International Hospital in Pakistan. Only 17 of these patients had a compatible donor while the remaining 14 patients did not. The Stanford algorithm for LPD identified an additional 5 patient-donor pairs (29.4% increase) while maintaining priority based on MELD scores.
Practical, Ethical and Operational Concerns
Although an efficient algorithm is essential for establishing LPD, there are several practical and logistical challenges to consider.
The general public in Pakistan has a poor perception of paired donations. While many donors are amenable to donating to a loved one, some are uncomfortable donating to strangers, especially if there is a risk of lobe mismatch. While the Stanford algorithm addresses the issue of inequity from lobe mismatching, more should be done to increase donor comfort through transplantation. We hope to adopt a multi-disciplinary approach that incorporates social workers, psychologists, and clergy to educate patients and provide psychosocial support. We expect that this clinical support, in addition to publicization of successful exchanges, may help shift perceptions of paired LDLT.
Donor safety is a priority for all LDLT transplant centers. Although left lobe resection is safer than right lobe resection, both carry a non-zero mortality risk.7,9 The risk to donors makes transplant centers and policy-makers reluctant to venture into LDLT, let alone LPD. Recently, however, attitudes have begun to shift as the mortality risk has dropped with technological improvements, increased experience, and meticulous donor selection.
A logistical obstacle to implementing LPD is donors backing out after other donors have already undergone surgery. To avoid this, donor operations need to be conducted simultaneously and require precise coordination between multiple transplant centers that may not be geographically proximate. Legal barriers present another challenge to incorporating LPD. In Pakistan, the Transplantation of Human Organs and Tissues Ordinance of 2007, which was signed into law in 2010, was designed to curb kidney trafficking by banning the sale of organs and allowing only close relatives of transplant recipients to serve as donors, except under special circumstances.11 As a result, nearly all LDLT in Pakistan involve donors that are blood relatives of the recipient. Since LPD may involve multiple non-relative exchanges, Pakistan’s Human Organ Transplantation Authority (HOTA) would have to explicitly allow paired donations for LDLT.
It is critical to differentiate compensated organ donation, which the Ordinance suppresses, from voluntary organ-paired donations, a technology that increases safe access to organs while reducing black markets demand. Several countries, including Islamic nations, use paired donations for kidney transplants, meaning HOTA can reasonably use the precedent set by these countries as regulatory bases to approve LPD. LPD would allow an additional one-third of all patients on the transplant list in Pakistan to receive a liver (Figure 2). Our team is actively advocating for regulatory reform on this issue with HOTA as we believe that denying patients this opportunity would be a true ethical hazard.
We believe there is stakeholder interest in promoting safe and accessible organ transplantation, as demonstrated by past advocacy from government, medical associations, international organizations, and national media, to facilitate the passage of the Ordinance in 2007. One of our co-authors (FD) is a pioneer for liver transplantation in Pakistan, setting up nearly every major transplant center in the nation as well as training teams in Quaid-e-Azam International Hospital, Shifa International Hospital, and the Pakistan Kidney and Liver Institute. With his involvement we are fortunate to have a coordination and mobilization point for establishing a nationwide liver exchange program using the Stanford Algorithm.
Conclusion
As the COVID crisis began (February to April 2020) in the United States, deceased-donor organ transplantations dropped 48%, living donor organ transplantations fell by 89%, and waitlists for organs grew by 1000-per-week.12,13 Similar patterns were observed worldwide as transplant activity ground to a halt, resulting in a backlog of demand for organs. Deceased donor transplantation, although essential, will not be enough to meet this increased demand as transplant centers resume services. The pandemic necessitates maximal utilization of the pool of living donors. We believe that using the Stanford algorithm for LPD does just that while also prioritizing donor safety and patient parity, and we hope that Pakistan’s effort in pioneering liver exchange will serve as a model for communities around the world, rich or poor.
About the Author
Dr. Saad Salman, MD, MPH, is an internal medicine resident at St. John's Riverside Hospital, New York, USA with a background in population health and interest in utilizing technology to establish broader population healthcare in lower socioeconomic demographics.
Sarah Gurev is a PhD student at the MIT Computer Science and Artificial Intelligence Laboratory (CSAIL) and a Richard H. Frazier (1923) Fellow.
Dr. Muhammad Arsalan, MBBS, FCPS (Surgery), is a Fellow at the Department of Liver Transplantation and the Department of Hepatobiliary & Pancreatic Surgery, Quaid-e-Azam International Hospital, Islamabad, Pakistan.
Dr. Faisal Dar (Sitara e Imtiaz), FCPS, FRCS, FEBTS, is the pioneer of liver transplantation in Pakistan. He is the Clinical Lead for New Life Health Services at Quaid-e-Azam International Hospital, Islamabad, Pakistan. He is the leading liver transplant surgeon in Pakistan, and he has successfully completed more than 1200+ transplant surgeries in his career.
Alex Chan, MPH, MS, is the Gerhard Casper Stanford Graduate Fellow at Stanford School of Medicine. He has research interests in market design, experimental economics, productivity in the healthcare sector, and the application of IT and machine learning in health policy. His email is chanalex@stanford.edu, and his Twitter handle is @alex8chan.
References
- Chan A. Liver Exchange with Equipoise. Stanford Health Policy Working Papers. 2020.
- Roth, A.E., Sönmez, T. and Ünver, M.U., 2004. Kidney exchange. The Quarterly journal of economics, 119(2), pp.457-488.
- Rees, M.A., Dunn, T.B., Kuhr, C.S., Marsh, C.L., Rogers, J., Rees, S.E., Cicero, A., Reece, L.J., Roth, A.E., Ekwenna, O. and Fumo, D.E., 2017. Kidney exchange to overcome financial barriers to kidney transplantation. American Journal of Transplantation, 17(3), pp.782-790.
- Wallis, C.B., Samy, K.P., Roth, A.E. and Rees, M.A., 2011. Kidney paired donation. Nephrology, Dialysis, Transplantation: Official Publication of the European Dialysis and Transplant Association-European Renal Association, 26(7), pp.2091-2099.
- Mishra, A., Lo, A., Lee, G.S., Samstein, B., Yoo, P.S., Levine, M.H., Goldberg, D.S., Shaked, A., Olthoff, K.M. and Abt, P.L., 2018. Liver paired exchange: Can the liver emulate the kidney?. Liver Transplantation, 24(5), pp.677-686.
- Hwang, S., Lee, S.G., Moon, D.B., Song, G.W., Ahn, C.S., Kim, K.H., Ha, T.Y., Jung, D.H., Kim, K.W., Choi, N.K. and Park, G.C., 2010. Exchange living donor liver transplantation to overcome ABO incompatibility in adult patients. Liver transplantation, 16(4), pp.482-490.
- Lee, S.G., 2010. Living-donor liver transplantation in adults. British medical bulletin, 94(1), pp.33-48.
- Ergin, H., Sönmez, T. and Ünver, M.U., 2020. Efficient and Incentive‐Compatible Liver Exchange. Econometrica, 88(3), pp.965-1005.
- Hartmann, A., Fauchald, P., Westlie, L., Brekke, I.B. and Holdaas, H., 2003. The risk of living kidney donation. Nephrology dialysis transplantation, 18(5), pp.871-873.
- Roth, A.E., 2015. Who gets what—and why: The new economics of matchmaking and market design. Houghton Mifflin Harcourt.
- Bile, K., Qureshi, J., Rizvi, S., Naqvi, S., Usmani, A., Lashori, K. (2010). Human organ and tissue transplantation in Pakistan; when a regulation makes a difference. Eastern Mediterranean Health Journal, 16(Supp.), 159-166. doi:10.26719/2010.16.supp.159
- Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. The Lancet. 2020; 395: E95-E96.
- United Network for Organ Sharing. COVID-19 and solid organ transplant. Date: May 26, 2020. https://unos.org/covid/ (Date accessed: May 26, 2020).











For many physicians, there is a lack of accessibility to healthy coping mechanisms and opting to ask for help. How can policy through legislation and broader reorganization of societal standards help alleviate physicians’ mental health and burnout?